What is oxidase test?
How does the oxidase test work? The oxidase test helps in identifying bacteria producing cytochrome c oxidase.
It is a particular type of enzyme of the bacterial electron transport chain. Oxidase positive bacteria are classified as aerobic (uses oxygen as electron acceptor in respiration). On the other hand, oxidase negative bacteria can be anaerobic, facultative, or even aerobic.
They came out negative in oxidase test because they do not have cytochrome c oxidase. What is the purpose of the oxidase test? Oxidase test detects organisms like Pseudomonas, Pasteurella, Vibrio, Neisseria, and Brucella. A negative oxidase test detects the presence of Enterobacteriaceae. (1, 2)
What is the reagent in oxidase test?
There are various types of reagents used for oxidase test. These include the following:
- Kovacs Oxidase
- Gordon and McLeod
- Gaby and Hadley Reagent (2, 3)
Special Precautions
- When picking the colony, you should avoid using nickel-base alloy wires that contains iron and chromium. Using them could lead to a false positive result.
- Make sure you interpret the result within 10 to 30 seconds.
- Make sure that when you perform the oxidase test, you have to use 5% sheep blood agar or any medium that does not contain fermentable sugar. Carbohydrate fermentation could lead to acidification of the medium, which could lead to a false negative oxidase test. (2, 4, 5)
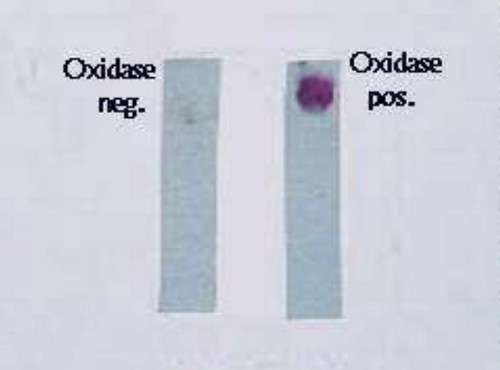
Photo 1: A comparison image of strips containing negative oxidase and positive oxidase
Picture Source: image.slidesharecdn.com
 Image 2: Oxidase test result using the test tube method
Image 2: Oxidase test result using the test tube method
Photo Source: www.medical-labs.net
 Picture 3: Oxidase test result using the plate method
Picture 3: Oxidase test result using the plate method
Image Source: www.uwyo.edu
How to perform oxidase test using different methods and their corresponding oxidase test results
Dry filter Paper Method
This is the most convenient way of performing oxidase test. To begin with, you will need a filter paper (#1) and soak in a 1% solution of tertramethyl-p-phenylene-diamine dihydrochloride. Drain for approximately 30 seconds.
Moisten the strip using a distilled water. Using the same strip, pick up the colony and smear over the moistened area. A positive result is characterized by changes in color (rich deep purple color), which appears within 10 seconds. If there is no changes in color, it means that the test is negative. (7)
Wet Filter Paper Method
The filter paper should be soaked in a 1% reagent solution. Using a platinum loop, the culture is rubbed on the filter paper. If the color changes into a rich deep purple within 10 seconds, it means that the result is positive. No changes in color yields to a negative result. (5)
Direct Plate Method
Using the reagent of your choice, put at least two to three drops of reagent to the bacteria colony. Make sure that the reagent solution you put is just enough to cover the colony. A positive result should lead to changes in color in a matter of 10 seconds.
Make sure that if you are going to use the direct plate method of oxidase test, it should be performed on a non-selective agar plate. Interpreting results using different reagents:
Kovac’s oxidase reagent
A positive oxidase test is confirmed if there is a noticeable change in color in a span of 10 seconds. A delayed oxidase positive is noted when the changes in color takes place within 60 seconds to 90 seconds. If there is no color change at all, the oxidase test is negative.
Gordon and McLeod
A positive oxidase test result is when the color of the culture changes to red in a span of 10 to 30 minutes. If the color changes to black in a span of 60 minutes, it is still an indicative of a positive oxidase test. If there is no significant change in color, then the oxidase test is negative. (5, 8, 9)
Swab method
This is one of the fastest methods. All you need to do is to submerge the swab into the reagent and touch the isolated suspect colony of bacteria. The changes in color should take place in a matter of 10 seconds. No significant change means a negative oxidase test.
Test Tube Method
This method takes some time, but yields to accurate result. You should grow a fresh culture of the suspected organism in a 4.5 ml of nutrient broth. It usually takes about 18 to 24 hours. Make sure that the broth does not have a high sugar content. Add about 0.2 to 0.3 ml of reagent, the ones usually used are Gaby and Hadley.
Mix thoroughly to see to it that the mixture is properly mixed and the culture has undergone a thorough oxygenation. A positive oxidase test is observed if the color of the culture changed to blue within 30 seconds.
A delayed positive oxidase test is noted if the color changed to violet in a span of three minutes. No notable change in color means a negative oxidase test. (10, 11)
Examples of oxidase positive organisms include the following:
- Pseudomonas
- Campylobacter
- Neisseria
- Vibrio
- Alcaligens
- Helicobacter pylori
- Moraxella
- Aeromonas
- Legionella pneumophila
- Pasteurella
- Brucella (6, 7, 9)
Example of oxidase negative organism
Enterobacteriaceae (e.g. E. coli)
Are there any limitations of oxidase test?
- Make sure you use fresh reagents (not older than one week) because reagents used for oxidase test oxidize on their own.
- Make sure that when growing the organisms, you should not use high glucose concentration because it can inhibit oxidase activity.
- Do not use media containing dyes as it could lead to inaccurate results.
- Organisms positive for oxidase test should be checked by a gram stain to find out the gram reaction and morphology.
- Use only platinum loops. Loop containing iron such as nichrome could yield to false positive result.
- When using the test tube oxidase test method, you should use colonies that are 18 to 24 hours old. A weak reaction is noted if you are going to use older colonies. (1, 2, 6, 10)
References:
- microbeonline.com
- www.microbiologyinfo.com
- https://en.wikipedia.org
- www.vumicro.com
- www.austincc.edu
- www.quora.com
- www.sigmaaldrich.com
- www.ncbi.nlm.nih.gov
- www.bacteriainphotos.com
- District Laboratory Practice in Tropical Countries, Part 2 By Monica Cheesbrough
- Koneman’s Color Atlas and Textbook of Diagnostic Microbiology edited by Elmer W. Koneman
